On America’s first Fourth of July in 1777, there was one color – orange. Fireworks have come a long way since then, taking on new colors and various shapes and sizes.
However, there is a lot of science behind these modern-day marvels.
What You Need To Know
- Fireworks are chemical reactions made of high energy compounds
- The type of atom used in the firework mixture gives off certain colors when heated
- Atmospheric conditions play a critical role in firework visibility
How it works
The colors that we see lighting up the night sky are caused by chemical reactions. The compounds in the firework are heated. These hot atoms give off light and that's what we see.
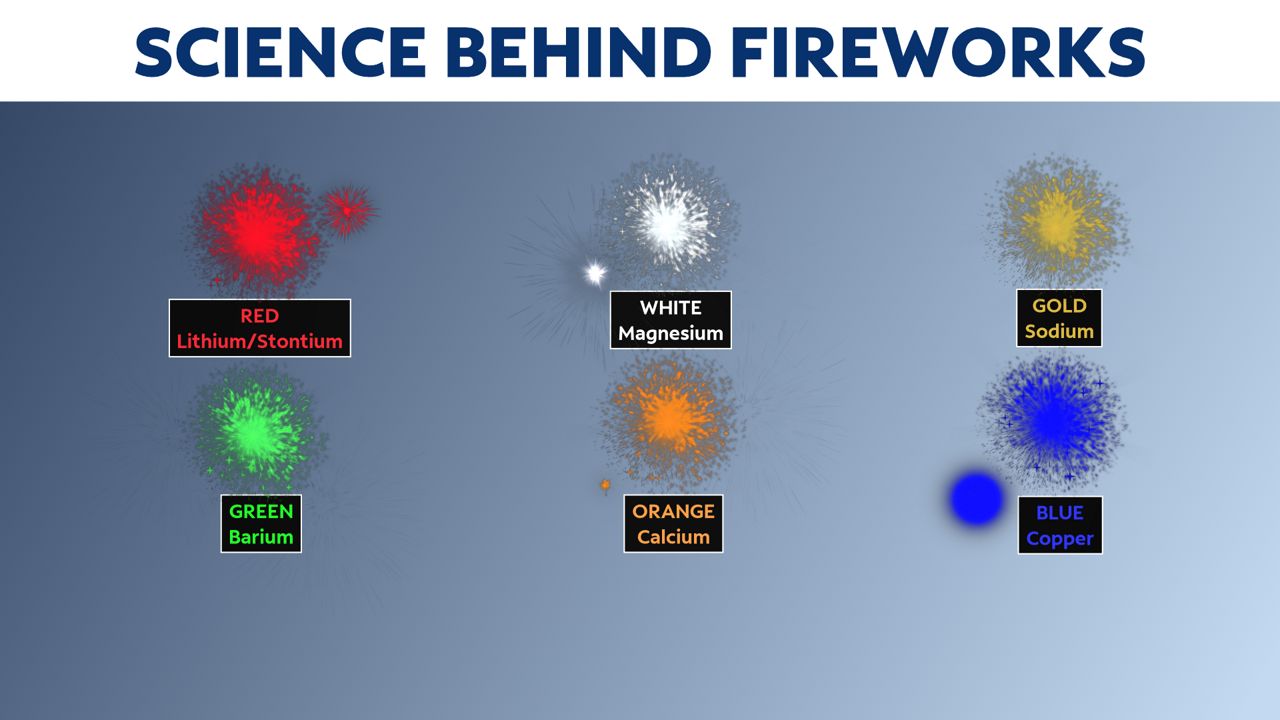
Different elements from the periodic table give off different colors. Lithium or strontium create a red color. Magnesium sparks a white color. Copper ignites a blue color.
The weather's impact
Weather can make or break a fireworks show.
It is a delicate balance when it comes to wind. Gusty conditions can be very problematic and blow debris onto spectators.

Meanwhile, light winds can also be an issue as there is nothing to help disperse the smoke.
Humidity is also very important. The lower the humidity the brighter fireworks appear. On muggy nights, fireworks can look dimmer and more muted.
Our team of meteorologists dives deep into the science of weather and breaks down timely weather data and information. To view more weather and climate stories, check out our weather blogs section.